Common examples are shaving cream and marshmallows. Aerosol contains small particles of liquid or solid dispersed in a gas.

Solutions Suspensions And Colloids
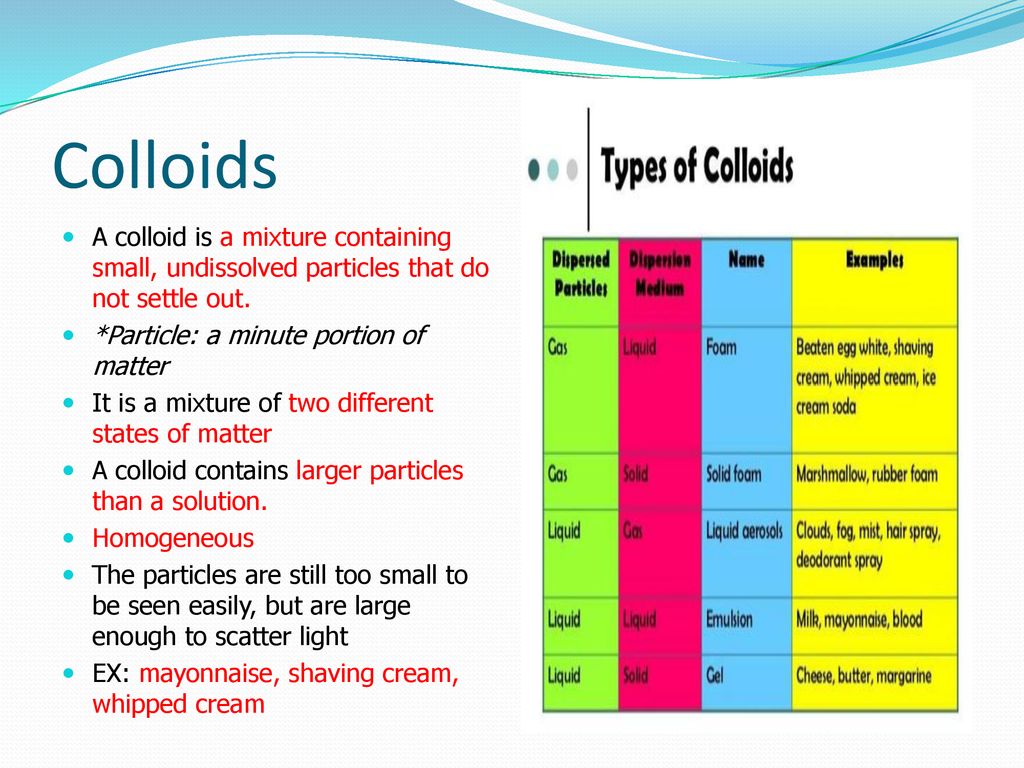
Because of such unique characteristics shaving cream and similar composite products are called colloid materials Generally speaking the colloid is the mixture of two or more different states of matter in which they do not dissolve in each other but remain discrete and reasonably stable.
. Even though the particles in a colloid are very small in size they can be seen through a process called the Tyndall Effect the effect of light scattering in colloidal dispersion while showing no. The colloid solution appears to be homogeneous but it is a heterogeneous mixture as well. B Gatorade sports drink.
Yeswhipped cream is a colloid because the substances neve dissolve into each other like a solution and the substances never settle out eventually and mix togethersuch other exmples would be milk. Get solutions Get solutions Get solutions done loading Looking for the textbook. A colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter yet are still able to remain evenly distributed throughout the solution.
A true solution is a homogenous mixture. Examples of colloids are foams shaving cream Styrofoam gels gelatin jelly emulsions mayonnaise lotion aerosols fog insecticide spray smoke and sols shampoo gemstones. Shaving soap is a solid.
It is a bonding of an Na ion and a Cl- ion. Shaving cream is a colloidal solution of A colloid is one of the three primary types of mixtures with the other two being a solution and suspension. C Kaopectate a medication used to treat diarrhea.
Neutral Atoms lon or Isotope. Which of these ions is bigger. Shaving lather whipped cream.
Other colloids may be opaque or have a slight color. Suspensions are heterogeneous mixtures. Due to the smaller size of milk particles it appears to be homogeneous but it is a heterogeneous mixture and such mixtures are called colloids or colloidal solutions.
Regular table salt is sodium chloride NaCl. It has two components solute and solvent. A colloid is a heterogeneous mixture.
It can be of three types- true solution suspension and colloids. The size of the particles of the solution is 1nm so they cannot be seen by naked eyes and do not show the Tyndall effect. It forms a false solution known as a colliod.
Shaving is a mixture of a gas dispersed throughout the cream. Shaving cream is a colloid in which dispersed phase is. Shaving cream in a can is a compressed liquid.
Colloids on the other hand have particles large enough to reflect light. A solution is a mixture of miscivle substances. These particles spread uniformly throughout the mixer.
Learn more about colloids at. One easy way to distinguish colloids from solutions is to shine a light through them. Solutions for Chapter 8 Problem 2P.
Colloids may be translucent due to the Tyndall effect where light is scattered by particles in the mixture. Foam is formed when many gas particles are trapped in a liquid or solid. Classify each product as a solution colloid or suspension.
Stay updated with the General Science questions answers with Testbook. As it appears in periodic table Atom Neutral lon or Protons Isotope Neutrons. Because the molecules in a solution are very small usually single molecules they dont reflect light.
They are stable in nature.

Solutions Colloids Suspensions Chemistry Lessons Science Lessons Matter Science

Question What Is The Difference Between Solutions Colloids And Suspensions Seniorcare2share
0 Comments